Moxifloxacin�� 莫西沙星�
Registration trends with CFDA
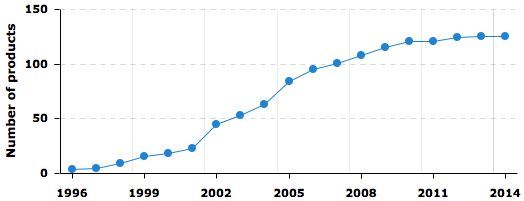
Add here a title
Total
Currently valid
Marketing approved registrants
2
2
Formulated products only
1
1
API Only
0
0
Both formulated product and API
1
1
Branded products
1
1
First registrant:
Lunan Hope Pharmaceutical Cp, Ltd.
First registration date:
2002
9 approved since 2014
2 expiring in 2015
Registration details or other title
| Approved companies | Brand Name/trademark | Formulation | First registration | Latest registration | Import registration | Domestic registration |
|---|---|---|---|---|---|---|
| 国食药监安[2005]624号 | Avelox 滴眼剂� | 2002� | 2014/12/20 | 23 | 0� | |
| 国食药监安[2005]624号 | 滴眼剂� | 五官科用药� | 2013/12/20� | 0 | 2� | |
| 国食药监安[2005]624号 | 滴眼剂� | 五官科用药� | 2013/12/20� | 0 | 2� |
Hi Guys !
In this second card is the content of registration in FDA. It should be displayed only when you click on registration FDA tab link, instead of CFDA content.
- Pierre 11/13/14
Number of approved companies
2
Number of unique registered brand
2
Number of registration companies in past one year
0
Number of registration companies in past five year
1
First registration date: 1999
| Approved companies | Brand Name/trademark | Formulation | First registration | Latest registration | Import registration | Domestic registration |
|---|---|---|---|---|---|---|
| 国食药监安[2005]624号 | Avelox 滴眼剂� | 2002� | 2014/12/20 | 23 | 0� | |
| 国食药监安[2005]624号 | 滴眼剂� | 五官科用药� | 2013/12/20� | 0 | 2� | |
| 国食药监安[2005]624号 | 滴眼剂� | 五官科用药� | 2013/12/20� | 0 | 2� |
